The Energy of an Sp Orbital Will Be:
2s orbital was previously here in energy. This regulation is divided into two parts.

2 The Other Sp Orbitals Hold The Oxygen Lone Pairs
Fluroine has 5 electrons in 2p orbital which means one degenerate orbital is partially filledoxygen on the other hand has two two such 2p orbitals partially fillednow partially filled orbitals which contribute to paramagnetism also.
. This makes HCN a Linear molecule with a. When a single p orbital contains a pair of electrons the act of pairing the electrons raises the energy of the orbital. Solved The energy of an sp orbital will be.
Each hybrid orbital is known as an SP 3 hybrid orbital. As the n in cespn grows larger we will have more p character and less s character in the hybrid orbital and as explained above it will consequently be higher in energy. It entails mixing 1 s orbital and p orbitals of the same energy to present a brand new hybrid orbital called sp 2.
In the example of ceN2 it is essential to bear in mind that each sp wont form an sigma and an sigma orbital. The significance is that the energy gap between the hybrid orbital eg. Hence s -s bond is non directional.
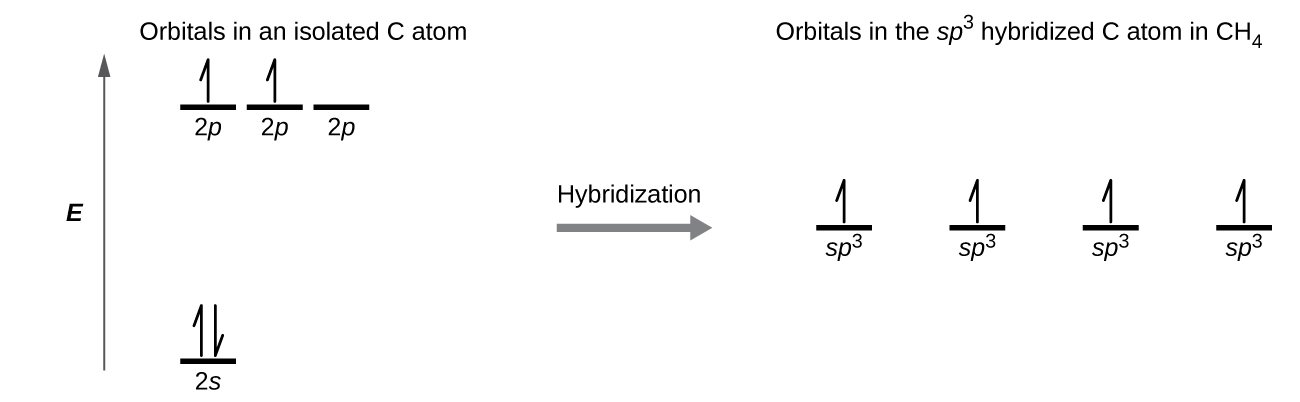
Due to this mixing One previously pure 2p orbital from above is lowered in energy to form an sp orbital. In an sp3 hybridization colorredone s orbital is mixed with colorredthree p orbitals to form colorredfour sp3 hybridized orbitals. Sp hybridization is observed when one s and one p orbital in the same main shell of an atom mix to form two new equivalent orbitals.
It forms linear molecules with an angle of 180. The sum of the principle quantum number n and the azimuthal quantum number a determines the energy of an orbital l. When they are bonding orbitals they.
So an cesp hybrid orbital that has 50 s character will be lower in energy than an cesp3 orbital which only contains 25 s character. Less than that of an s or p orbital greater than that of an s or p orbital less than that of an s orbital but greater than that of a p orbital less than that of an p orbital but greater than that of a s orbital. S-p mixing occurs when the s and p orbitals have similar energies.
It is half-filledTwo such 1s orbitals from the two hydrogen atoms having electrons with opposite spins approach each other then the potential energy of the system. Sp 2 hybridization is likewise referred to as trigonal hybridization. In each SP 3 hybrid orbital the S character is only 25 while the P character is 75.
The energy of an sp orbital will be. This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as an sp hybridized orbital. The energy of an sp orbital will be.
S orbital is spherical in shape and overlapping takes place to some extent in all directions. Therefore these 4 SP 3 hybrid orbitals will not be very close to the nucleus as they will be. Molecular orbitals are formed from the overlap of hybridized and from unhybridized orbitals in all possible permutations.
One previously pure 2s orbital rises in energy a bit to form an sp orbital. Each sp hybridized orbital has an equal amount of s and p character 50 s and 50 p character. When we have an sp hybrid orbital it is usually made of the s orbital and the p orbital that points in the bounding axis p_z.
These sp3 hybridized orbitals are oriented with bond angle of. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. This is called the nl rule.
The Hybridization in which one S and three P orbitals intermix and form four hybrid orbitals of the same shape and energy is known as SP 3 Hybridization. When Carbon is bound to two other atoms with the help of two double bonds or one single and one triple bond. Answer 1 of 2.
Sp hybridization is also called diagonal hybridization. Answer 1 of 3. Chemistry questions and answers.
Less than that of a p orbital but greater than that of an s orbital An sp orbital will have an energy in between those of the unhybridized s and p orbitals. Thus the 2p orbitals for O F and Ne are higher in energy than the 2p orbitals for Li Be B C and N. What is SP mixing in molecular orbital theory.
Its energy will be the mean of the energy of the initial orbitals. Hydrogen 1s 1 atom has 1s orbital containing a single electron ie. When atomic orbitals hybridize the valence electrons occupy the newly created orbitals.
When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. In a one electron atom hydrogen or hydrogen-like atomion they all have the same energy within a principle n group. Sp and sp 2 and an unhybridized p orbital is smaller than the spin paring energy and therefore electrons will be in the hybrid and unhybridized p orbital state before.
In a more usual atom with multiple electrons then you have to take into account electron-electron repulsion. Both C and N have 2 p orbitals each set aside for the triple bond 2 pi bonds on top of the sigma. The new orbitals formed are called sp hybridized orbitals.
Choose one -less than that of an s or p orbital -greater than that of an a or p orbital -less than that of an s orbital but greater than that of a p orbital -less than that of a p orbital but greater than that of an s orbital. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Which has the highest energy among hybrid unhybrid and molecular orbital.
The energy of an sp orbital will be. A Orbitals with a lower n l value have less energy than orbitals with a greater. A mixture of s and p orbital is shaped in trigonal symmetry and is maintained at 1200.
Each of these hybridized orbitals have 25 s character and 75 p character calculated according to the proportion of sp mixing. N has one sigma bond to C and the other sp hybrid orbital exists for the lone electron pair.
File Sp Orbital Energy Diagram K Svg Wikimedia Commons

Comments
Post a Comment